Prime Time

“Go big or go home” is a phrase I often use when describing RSRT’s research philosophy. Our goal, as many of you know, is to attack Rett Syndrome at its very core: MECP2 mutations. There are a number of ways to achieve this. Traditional gene therapy, or "gene replacement" as it’s often being referred to, is one way. Extra copies of MECP2 are delivered into cells via a viral vector. This strategy however, which is the closest to the clinic, doesn't allow us to control how many copies of the gene enters any particular cell. There is the possibility that too many copies of the gene could cause a problem.
A more elegant approach that would eliminate overexpression concerns would be to edit mutations within the MECP2 gene. You can think of DNA editing as molecular tweezers that literally swaps the mutated DNA for the correct DNA. Among the groups we are funding in this space is Beam Therapeutics. David Liu is one of the cofounders of Beam Therapeutics and maintains his lab at the Broad Institute in Cambridge, MA. You may have heard of a significant breakthrough in DNA editing called Prime Editing. Below is an explanation of how Prime Editing works and how it may be able to repair up to 89% of mutations that cause disease.
Let’s review a few basics first. CRISPR is a protein that can be programmed to recognize a specific sequence of DNA. The recognition is done by a small piece of RNA aptly called a guide sequence, which leads the CRISPR protein to the correct site on the DNA. In order for the CRISPR to bind to the DNA there is a requirement for a specific sequence of DNA bases within close proximity to the mutation that has to be corrected. That sequence is called a PAM sequence and its requirement limits the number of mutations that can be repaired by the current base editors.
Once the CRISPR protein finds the correct site it helps unwind the DNA so the guide sequence can bind. The CRISPR protein, called CAS9, originally cut DNA in half. It has subsequently been improved so it only nicks the DNA. These improvements allow for greater reliability in the edits.
These improved versions of CRISPR CAS9 also have other proteins attached to them that can modify DNA. These “fusion” CRISPR proteins are called base editors and they can perform very specific edits. However there are limitations in the number and types of mutations they can edit. They are ideally suited for particular point mutations (C>T, T>C, A>G, G>A) that have a PAM sequence within close proximity.
In situations where the PAM sequence is not ideally situated then PRIME editors are better options. Importantly, PRIME editors can edit other classes of mutations beyond point mutations such as insertions, deletions, and the eight other kinds of point mutations.
To date Prime Editing has only been achieved in cells. Next steps will be to take this technology into animals. This is a field that is moving very quickly and David Liu has a track record of making serial breakthrough advances so I have every expectation that progress will be made.
I saw Dr. Liu present these data at a recent meeting in Boston and had an opportunity to discuss with him the business relationship between Prime Medicine and Beam Therapeutics. The good news is that our existing relationship with Beam Therapeutics will give us access to this technology through a sublicense to Beam from Prime Medicine.
I look forward to keeping you updated on developments!
For the fellow science nerds out there below is more information on how Prime Editing works.
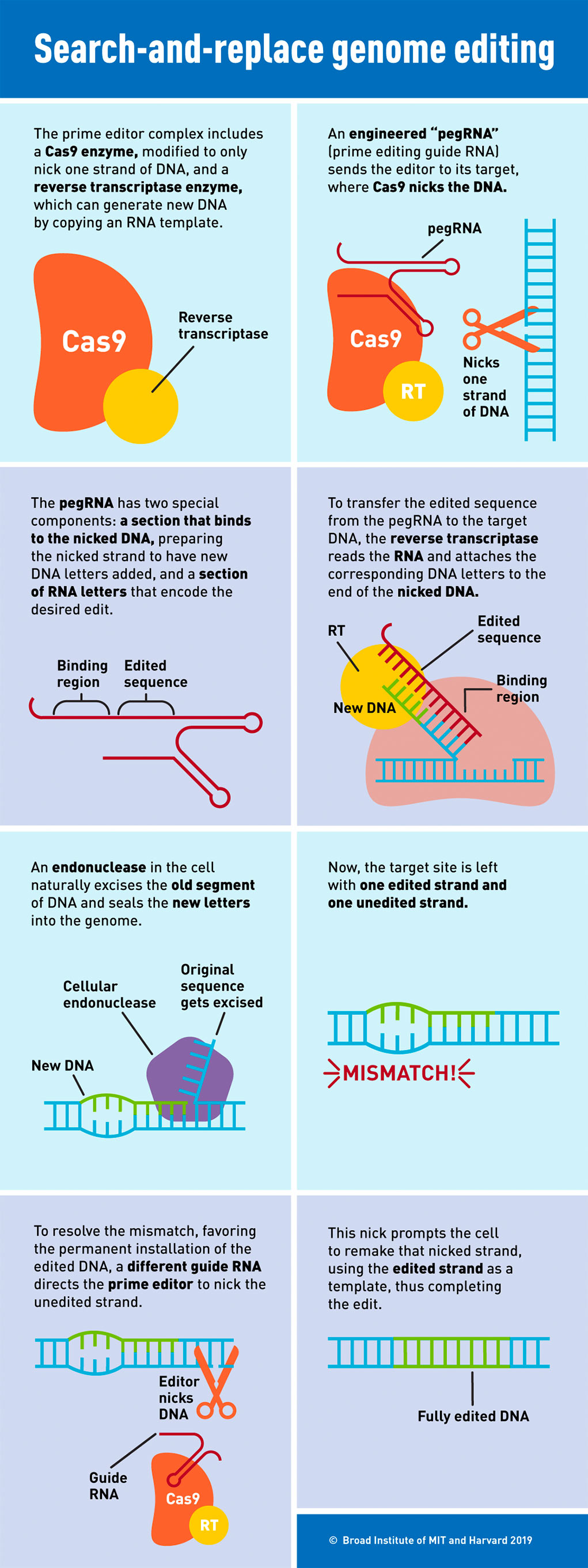
In the case of Prime Editing there are two extra pieces added to the CRISPR CAS9 molecule. One is a different protein called Reverse Transcriptase or RT for short (see the first block in the figure below). RT is an enzyme that reads single stranded RNA and turns it into double stranded DNA. Normally CRISPR cuts the DNA in half. However, this new type of CRISPR protein only clips one of the strands of the DNA (see block 2). The other piece that is added is another stretch in the guide sequence that is actually carrying the information to repair the mutation in the MECP2 protein (see block 3). This information is now read by the RT protein, turned into the correct stretch of DNA that repairs the mutation in the MECP2 (see block 4). The natural repair machinery in all of our cells now steps in and clips out the bad sequence from the MECP2 DNA code and reconnects that nicked strand of DNA (see block 5). This leaves a bulge in the DNA (see block 6) which is smoothed out by another nick in the opposite strand leaving the repaired DNA (see block 8).
Reference:
The Harvard Gazette, Karen Zusi, 21 Oct 2019