New Insight Into How MeCP2 Regulates Transcription

Since our previous blog post on the RSRT website, we have made significant progress toward understanding an important function of MeCP2: how it regulates gene transcription. We wanted to share this exciting new research with the RSRT community. We hope and expect that this research, which is funded by RSRT and the NIH, will help guide therapeutic approaches that could improve the lives of girls with Rett syndrome. And as ever, we would like to express our ongoing gratitude to RSRT and its supporters.
RSRT MECP2 Consortium member Adrian Bird discovered over 25 years ago that MeCP2 binds to methylated DNA and that it helps repress gene expression. However, we as a research community have had a difficult time understanding which genes MeCP2 actually regulates and how it regulates them. This difficulty arises because MeCP2 binds broadly across the genome rather than at a few specific places and because the changes in gene expression associated with MeCP2 dysfunction are quite small. Despite these challenges, our laboratory and others recently found that the genes upregulated when MeCP2 is mutated have two special characteristics: 1) they have high levels of gene body DNA methylation and MeCP2 binding and 2) they are very long. These findings inspired the hypothesis that MeCP2 bound within gene bodies might function as a sort of “speed bump” to RNA Polymerase II (Pol II), the enzyme that transcribes genes into RNA. According to this hypothesis, if MeCP2 function were disrupted, the genes that normally have MeCP2 bound in their gene bodies would be transcribed more quickly.
We tested this hypothesis in our recently published article in Molecular Cell by developing a new method that allowed us to directly measure how quickly genes are transcribed in the mouse brain. To our surprise, we did not find significant differences in the rate of gene transcription in MeCP2-mutant mice compared to wild-type mice. Instead we found that MeCP2 regulates how quickly Pol II can be recruited to the beginnings of genes, a process called transcriptional initiation. Moreover, the increase in transcriptional initiation we observed in MeCP2-mutant mice was greatest for the genes that are highly methylated and long. These findings suggest that therapeutic approaches that selectively attenuate transcriptional initiation of highly methylated long genes might be useful in treating Rett syndrome.
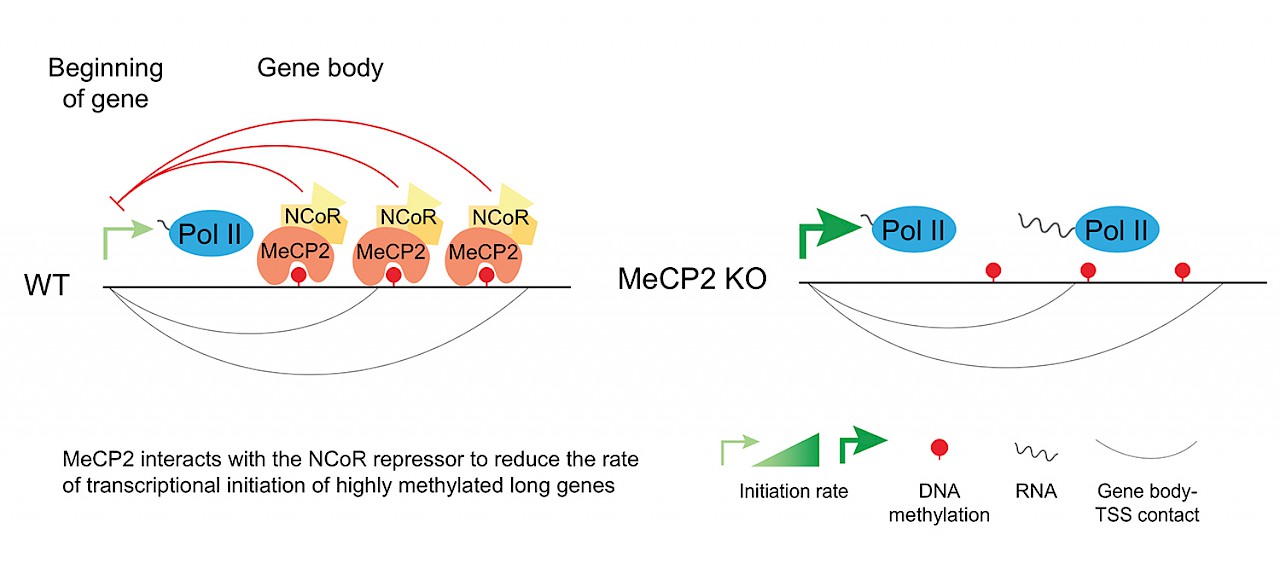
The finding that MeCP2 represses transcriptional initiation was surprising because there is much less MeCP2 present at the beginning of genes than there is over the rest of the gene body. However, we found that there are three-dimensional DNA contacts between the gene body and the beginning of genes, suggesting that MeCP2 in gene bodies might act from a distance through these contacts to repress transcriptional initiation. Interestingly, an article that was co-published with ours found that MeCP2 represses transcription by repressing gene body enhancers, specific regions of DNA that can act from a distance to enhance transcription of genes. Ongoing studies in our lab are further exploring these new MeCP2 mechanisms to uncover the direct molecular function of MeCP2 and the genes it regulates. Our hope is that improved understanding of the direct function of MeCP2 will inspire novel therapeutic strategies for Rett syndrome.
This research couldn’t happen without RSRT and its supporters and all the families affected by Rett Syndrome that raise funds. We look forward to keeping you apprised of further progress.


